Reducing the Environmental Footprint of Your Healthcare Products with Data-Driven Eco-Design Strategies

Our Capabilities
Our approach and team working with Sustainability Engineering can help you with:
Sustainability in Product Development
Life Cycle Screenings and Assessments
Corporate Sustainability
Eco-Design Principles
Take-Back Systems
Regulatory Compliance
Material Selection
Device and Packaging
Absolute Sustainability
Gain Clarity and Assurance About the Effects of Your Sustainability Efforts
The medical device manufacturers we work with are seeing a steep increase in sustainability demands coming at them from every angle. Despite a general wish to share in the environmental responsibility and future-proof their businesses, setting the sustainability effort on a steady trajectory can be a major challenge for healthcare manufacturers.
Development cycles lasting several years and strict regulatory requirements to products and safety can make it difficult to incorporate sustainability into an already complex development process.
Make Informed Decisions About Sustainable Design Improvements
Our two-pronged approach provides a framework for making sustainability assessments and improvements from the very beginning and throughout the device development project. This is done by combining eco-design principles with Life Cycle Screenings and bringing important aspects from Life Cycle Assessments (LCAs) into the core of the healthcare product development process in a manner that is much faster and more agile than with traditional LCAs.
Rather than being retrospective in character like LCAs, our approach offers value during all stages of product development. The goal is to bring a proactive approach to sustainable product development by gaining fast access to dependable sustainability data throughout the development process.
We see that using eco-design principles when deciding on sustainable design improvements helps our clients to concentrate their effort on the changes that have the most impact. The eco-design principles are firmly grounded in industry best practices end therefore align with the sustainability guidelines of our clients. The principles are also based on established theory from the Eco-design research community, and they align with designing for a circular economy.
Our Life Cycle Screenings are based on the Life Cycle Assessment methodology in ISO 14040/14044 and provide similar information as an LCA, but based on generic data and assumptions. This makes it applicable during a product development process, providing invaluable feedback to the Ecodesign process in an iterative way. Whether your focus is sustainable packaging or medical device sustainability, our experts can help.

WANT TO KNOW MORE?
Get in Touch
Do you want to know more or didn’t you find what you were looking for?
Our team within Sustainability is ready to help.
We Have the Expertise to Take You From A to Z
Whether you need a single competency or a combination of skills, our experts in Design & Development are ready to assist you through the entire process. Based on the specific needs of the project, we assemble the ideal team of specialists.
We are able to integrate advanced manufacturing knowledge into the earliest design phase and minimize the number of iterations. Our complete offering takes your project seamlessly through the entire value chain – From Imagination to Realization.
Check out all our areas of early-stage design expertise.
Related Cases
How an FDA Pre-submission Enhances Your Usability Engineering Process
Discover the advantages of incorporating FDA pre-submission insights into your usability engineering process for streamlined success.
Application of Human Factors Engineering Principles for Combination Products
News from fda Application of Human Factors Engineering Principles for Combination Products — Questions and Answers
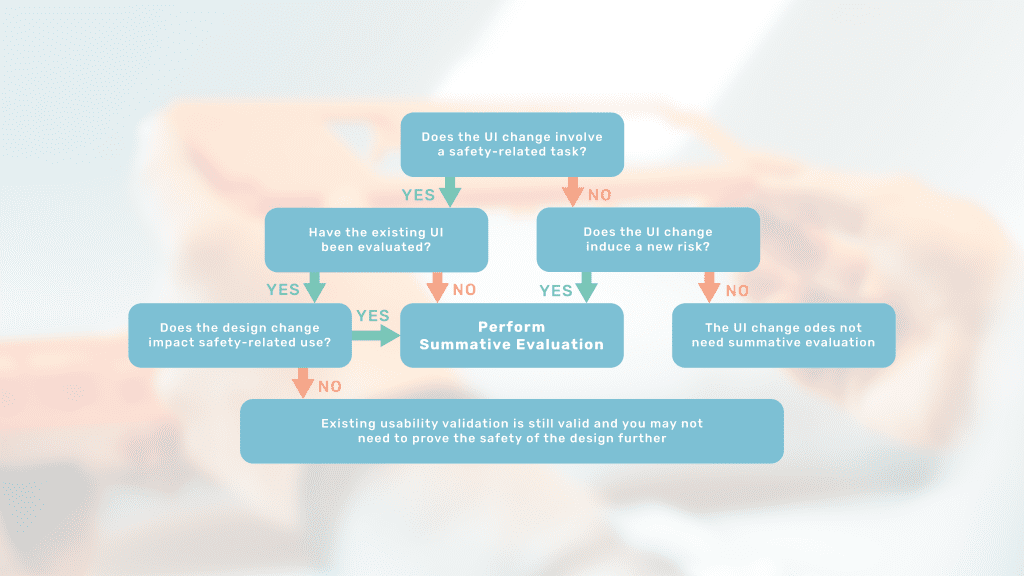
How Design Changes Impact Usability
This article will introduce you to assessing the usability impact of your design change and thereby plan for any needed summative evaluation.