Test and Validation Processes that Set Global Standards
A critical final step in the moldmaking process is final testing and validation against stringent industry standards to ensure the highest levels of quality and efficiency in the manufacturing process. Our global medical product engineering team uses expertise gained from decades of testing and validation programs to assess and document all critical performance data. We work with customers to test and validate these programs in both Europe and North America – driving efficiency closer to where they manufacture and distribute products.
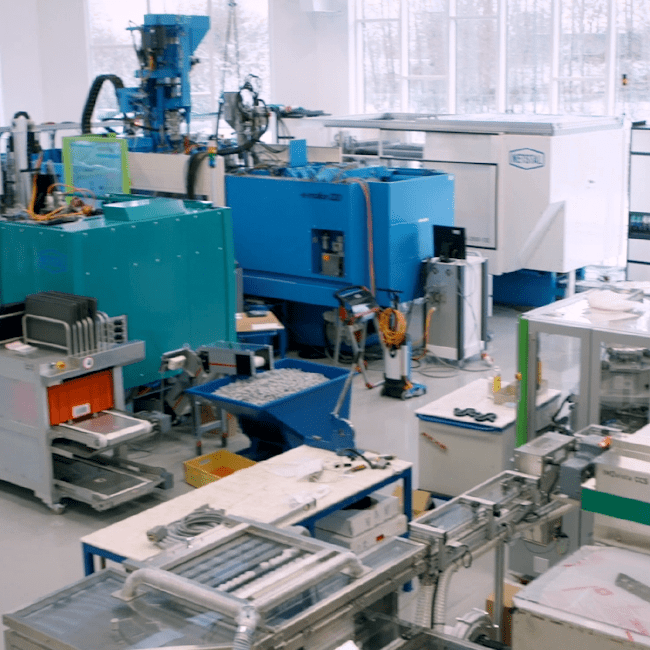
Test and Validation Support from Global Innovation Centers
In the U.S., construction is underway on a state-of-the-art 119,000ft2 Innovation Center that will offer dedicated test and validation space for North American customers.
Our Test and Validation Approach
We lead customers through process and product development to ensure the highest levels of quality while helping them get to market faster.
Mold Testing
We follow scientific molding principles to test high-precision healthcare molds for our customers. Our approach includes debugging, material tests, samples for automation testing, and mold Factory Acceptance Testing (FAT). Even our First Off Tool (FOT) samples are produced through a systematic process optimization with in-house 3D scanning, optical- and/or tactile CMM measurement and beyond. All testing is completed with proper documentation to streamline program approvals. We test in secure environments to guarantee confidentiality.
Pilot Production
We deliver a fully debugged solution to deliver validated pilot production ahead of scaling to full production volumes. We do so in ISO 13485-certified facilities and Class 7 and 8 cleanroom environments to fill your pipeline with components to meet your immediate needs.
Turnkey Manufacturing Cell Development
Within our dedicated test and validation centers, we build and validate turnkey manufacturing cells to drive quality improvements and cost optimizations. We deliver high OEEs of validated turnkey cells ready for high-volume production – either at your facility or a global MGS manufacturing site. After we transfer these turnkey cells to you, customers have access to history, know-how, 3D scan measuring and process data.
Worldwide Service and Support
We also offer worldwide service and support to validate programs with real-time support from MouldCat® and MouldBox® to monitor mold performance and enable predictive and preventative maintenance. We drive efficiency for our customers by offering virtual service using latest augmented reality techniques and conference systems – both on-site at any of our global Innovation Centers, or via Teams or similar conferencing systems.
Struggling with Tooling Development Timelines?
Contact MGS today to see how our proven processes can get you to market ahead of the competition.
