The U.S. Food and Drug Administration (FDA) offers manufacturers the opportunity to engage with the Agency before submitting their final submissions or applications, e.g. 510(k) submissions. Such interactions with the FDA are typically referred to as pre-submissions or Q-submissions. A pre-submission allows the manufacturer to get the FDA’s input before initiating larger activities, e.g., Summative Evaluation (Human Factors Validation Studies) and Clinical Studies. In their guidance, the FDA states, “A Pre-Sub includes a formal written request from a submitter for feedback from FDA that is provided in the form of a formal written response or if the submitter chooses, formal written feedback followed by a meeting.” The guidance is available here.
Case Stories
Recently, MGS Design & Development has been actively engaged in advising and supporting Pharma and MedTech manufacturers in pre-submissions where the following was in focus:
Foregoing Summative Evaluation
A Software as a Medical Device (SaMD) manufacturer presented and outlined potential harm categories associated with their device. By demonstrating that none of the identified use errors could result in serious harm as per FDA guideline (21 CFR 803.3(w)), the manufacturer successfully argued that their device didn’t entail critical tasks. Consequently, they were able to forego Summative Evaluation (Human Factors Validation Study) for their upcoming 510(k) premarket submission. This significantly reduced both time to market and the budget for development activities.
Self-Selection Strategy and Sample Size Confirmation
The Summative Evaluation (Human Factors Validation) study design – specifically the self-selection approach for an Over-the-Counter (OTC) medical device – and participant sample size were deemed appropriate by the FDA thereby resulting in neither human factors deficiencies nor questions for the final market submission.
Efficient Validation Strategies
Leveraging existing HF validation data on already marketed products to include new medical indications and forego new human factors validation studies.
Benefits of Pre-Submission
Why prioritize a pre-submission?
A frequent concern we hear is reluctance to engage the FDA early because a pre-submission might extend the timeline by 2–3 months. While a pre-submission can add time, it must be weighed against the risk of a final FDA submission being delayed or not accepted. From a Human Factors/Usability Engineering standpoint, issues that commonly surface include Summative Evaluation (Human Factors Validation) study design or methodology problems, such as:
- Use errors tied to identified critical tasks that are better assessed through actual or simulated-use scenarios rather than knowledge tasks.
- Omission of one or more user groups expected by the FDA.
- Participant recruitment that does not align with current demographics.
- Incorrect categorization of critical vs. non-critical tasks.
- Critical tasks omitted from the scope of the Human Factors Validation study.
These are only a few examples of the feedback FDA may provide. The guidance
Requests for Feedback and Meetings for Medical Device Submissions: The Q-Submission Program
lists sample pre-submission questions, including human factors.
When do we recommend a pre-submission?
- Your use-related risk assessment indicates no use errors with the potential to lead to serious harm, and you want FDA’s concurrence on the absence of critical tasks so you can forgo a Summative Evaluation (Human Factors Validation Study).
- There is uncertainty about the study design or methodology of your Summative Evaluation.
- The intended user group is highly restricted and difficult to recruit, and you plan to justify surrogate or proxy participants whose capabilities and limitations sufficiently mirror the intended users.
- You are seeking FDA feedback on bridging strategies to leverage existing human factors data.
- Getting it right on the first submission is more important than rigid adherence to internal timelines.
Conclusion
Engaging FDA through pre-submissions aligns expectations and streamlines later marketing submissions. Early identification of potential issues enables appropriate mitigations, reducing the risk of delay or rejection during the final submission. Contact us to discuss how a pre-submission can benefit your current or next project.

WANT TO talk?
Let’s get in touch
Our Senior Human Factors Specialist, Morten, is looking forward to discussing how an FDA pre-submission enhances your usability engineering process. Drop him a mail directly or give us a call. We are looking forward to hearing from you.
Insights you might also be interested in:
Application of Human Factors Engineering Principles for Combination Products
News from fda Application of Human Factors Engineering Principles for Combination Products — Questions and Answers
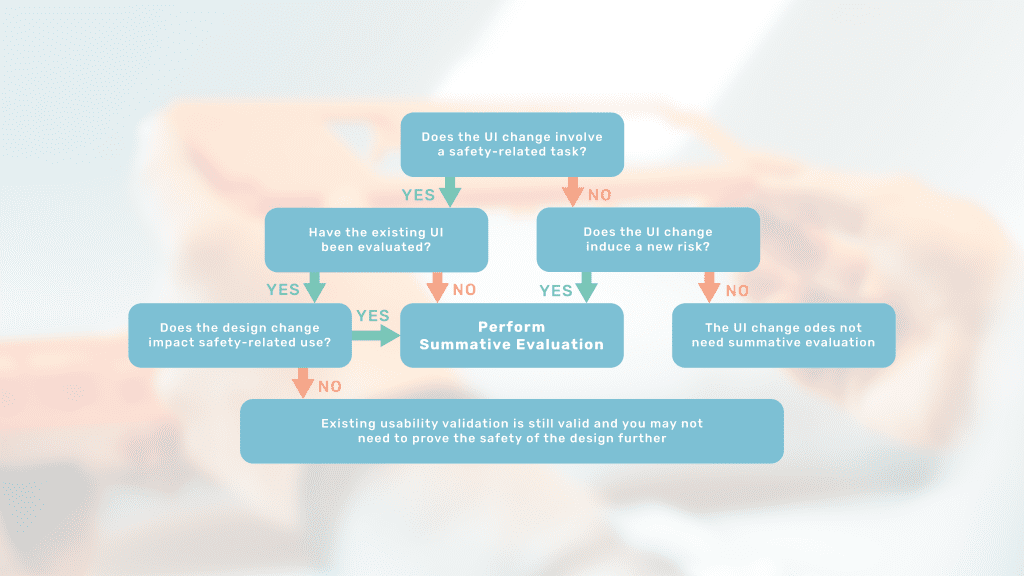
How Design Changes Impact Usability
This article will introduce you to assessing the usability impact of your design change and thereby plan for any needed summative evaluation.
Identifying Potential Use Errors
Find out how you can identify use errors and their sources and get valuable design input with PCA analysis.