Medical Device Usability Engineering Services for Safer, More Effective Products

Our Capabilities
Whether your organization needs a single area of our medical device Usability Engineering expertise or the entire range, we are here to help you.
Usability Early Research
Known Use Problems
Use Related Risk Analysis
Formative & Summative Testing
Heuristic Evaluations & Expert Reviews
Usability Documentation Review
Usability Submission Strategy
Usability Courses and Training
HFE Plan & Report
We Work With All Types of MedTech Development, Including Devices, IVD, Combination Products and Software
 It is not enough for a medical device to have the right features if users cannot use them properly. Designers must consider human abilities and limits during use. The user interface needs just as much focus as other functions during the medical device development process.
It is not enough for a medical device to have the right features if users cannot use them properly. Designers must consider human abilities and limits during use. The user interface needs just as much focus as other functions during the medical device development process.
What We Offer
MGS will support you in integrating usability into your device development process while thoroughly documenting each step to ensure regulatory compliance.
Our Usability Engineering services for Pharma, Diagnostics and MedTech cover the full process. We support early user research, simple user interface tests, final usability tests and HFE reports. We also help teams update existing products and make design improvements.
MGS Design & Development works in compliance with recognized Human Factors and Usability Engineering standards and guidance (FDA guidance & IEC 62366-1:2015).
Expert Knowledge and Extensive Experience
Our expert knowledge and extensive experience enable us to advise our clients on both the strategic and operational level. When working with Usability Engineering, we make sure to integrate the activities with Design Control and Risk Management. We use inputs from these functions to ensure our results deliver input for the User Interface Specification and Risk Analysis. Our in-house product development capabilities furthermore enable us to provide redesign suggestions to improve the usability of your Pharma, Diagnostic or MedTech device.
For user testing, MGS Design & Development can also provide test facilities and test recruitment assistance both in Europe and in the US. We also offer Usability courses, tailor-made to suit your company needs and product category.
Focusing on and prioritizing Medical Device Usability Engineering activities will generate numerous benefits:
- Minimized need for training and support.
- Fewer late-stage changes in development, ultimately leading to faster time-to-market.
- Simpler and more intuitive product user interfaces.
- Improved user satisfaction leading to improved sales.
- Improved market life and customer loyalty.
- Minimized risk of liability claims and product market withdrawals.

Want to know more?
Get in Touch
Do you want to know more or didn’t find what you were looking for? Send our Usability Engineering Team a message.
We Have the Expertise to Take You From A to Z
Whether you need a single competency or a combination of skills, our experts in Design & Development are ready to assist you through the entire medical Usability Engineering process. Based on the specific needs of the medical device project, we assemble the ideal team of specialists.
We are able to integrate advanced manufacturing knowledge into the earliest design phase and minimize the number of iterations. Our complete offering takes your project seamlessly through the entire value chain – From Imagination to Realization.
Check out all our areas of early-stage design expertise.
Related Cases
How an FDA Pre-submission Enhances Your Usability Engineering Process
Discover the advantages of incorporating FDA pre-submission insights into your usability engineering process for streamlined success.
Application of Human Factors Engineering Principles for Combination Products
News from fda Application of Human Factors Engineering Principles for Combination Products — Questions and Answers
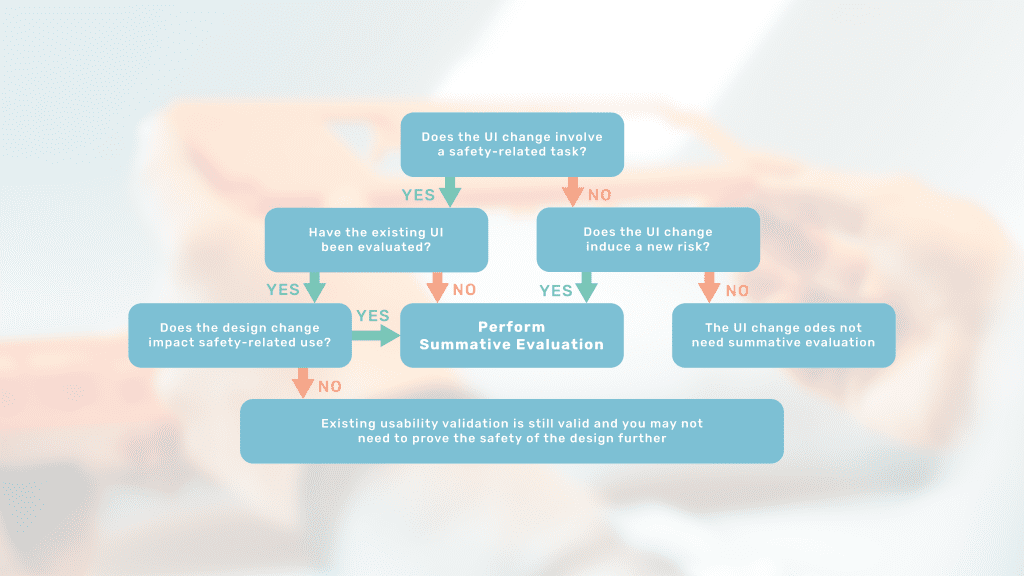
How Design Changes Impact Usability
This article will introduce you to assessing the usability impact of your design change and thereby plan for any needed summative evaluation.